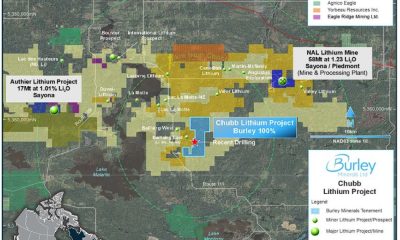
Energy & Critical Metals
US-China research team discovers reaction mechanism that can overcome rapid performance decline in lithium-sulfur batteries
Researchers from the US and China have identified a previously unknown reaction mechanism that addresses the very short lifetimes of Li-sulfur batteries.Their…
Researchers from the US and China have identified a previously unknown reaction mechanism that addresses the very short lifetimes of Li-sulfur batteries.Their paper is published in the journal Nature.
Lithium-sulfur batteries can store two to three times more energy in a given volume, resulting in longer vehicle ranges. Their lower cost, facilitated by the abundance and affordability of sulfur, makes them economically viable. Further, these batteries do not rely on critical resources such as cobalt and nickel, which may face shortages in the future.
Despite these benefits, transitioning from laboratory success to commercial viability has proven elusive. Laboratory cells have shown promising results, but when scaled up to commercial size, their performance rapidly declines with repeated charge and discharge.
The underlying cause of this performance decline lies in the dissolution of sulfur from the cathode during discharge, leading to the formation of soluble lithium polysulfides (Li2S6). These compounds flow into the lithium metal negative electrode (anode) during charging, further exacerbating the issue. Consequently, the loss of sulfur from the cathode and alterations in the anode composition significantly hinder the battery’s performance during cycling.
In a recent earlier study, Argonne scientists developed a catalytic material that, when added in a small amount to the sulfur cathode, essentially eliminated the sulfur loss problem. While this catalyst showed promise in both laboratory and commercial-size cells, its atomic-scale working mechanism remained an enigma until now.
The team’s most recent research shed light on this mechanism. In the absence of the catalyst, lithium polysulfides form at the cathode surface and undergo a series of reactions, ultimately converting the cathode to lithium sulfide (Li2S).
The presence of a small amount of catalyst in the cathode makes all the difference, said Gui-Liang Xu, chemist in Argonne’s Chemical Sciences and Engineering division. “A much different reaction pathway follows, one free of intermediate reaction steps.”
Different reaction pathways from lithium polysulfide (Li₂S₆) to lithium sulfide (Li₂S) in lithium-sulfur batteries with (left) and without (right) catalyst in sulfur cathode. (Image by Argonne National Laboratory.)
Key is the formation of dense nanoscale bubbles of lithium polysulfides on the cathode surface, which do not appear without the catalyst. These lithium polysulfides rapidly spread throughout the cathode structure during discharge and transform to lithium sulfide consisting of nanoscale crystallites. This process prevents the sulfur loss and performance decline in commercial-size cells.
Analyses of the catalyst’s structure with the intense synchrotron X-ray beams at beamline 20-BM of the Advanced Photon Source, a DOE Office of Science user facility, revealed that it plays a critical role in the reaction pathway. The catalyst structure affects the shape and composition of the final product upon discharge, as well as the intermediate products. With the catalyst, nanocrystalline lithium sulfide forms upon full discharge. Without the catalyst, microscale rod-shaped structures form instead.
Another vital technique, developed at Xiamen University in China, allowed the team to visualize the electrode-electrolyte interface at the nanoscale while a test cell was working. This newly invented technique helped connect changes at the nanoscale to the behavior of an operating cell.
Resources
-
Zhou, S., Shi, J., Liu, S. et al. (2023) “Visualizing interfacial collective reaction behaviour of Li–S batteries.” Nature 621, 75–81 doi: 10.1038/s41586-023-06326-8

Uranium Exploration Company Announces Additional Staking in the Athabasca Basin
Source: Streetwise Reports 12/22/2023
Skyharbour Resources Ltd. announced an update from its Canada-based Falcon Project along with additional…
Tesla Launches New Mega Factory Project In Shanghai, Designed To Manufacture 10,000 Megapacks Per Year
Tesla Launches New Mega Factory Project In Shanghai, Designed To Manufacture 10,000 Megapacks Per Year
Tesla has launched a new mega factory…
Giving thanks and taking stock after “a remarkable year”
An end-of-year thank you to our readers, industry colleagues and advertisers before Electric Autonomy breaks from publishing until Jan. 2
The post Giving…