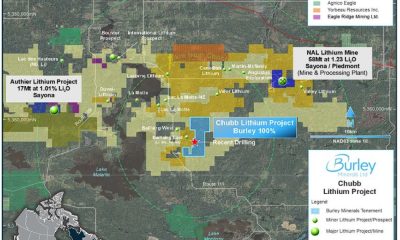
Energy & Critical Metals
New method to produce layered lithium cobalt oxide cathode materials at low temperatures; hydroflux process
Traditionally, the synthesis of layered lithium cobalt oxide cathode materials for Li-ion batteries requires temperatures above 800 °C and takes 10 to…
Traditionally, the synthesis of layered lithium cobalt oxide cathode materials for Li-ion batteries requires temperatures above 800 °C and takes 10 to 20 hours to complete. Now a team of researchers at Hokkaido University and Kobe University, led by Professor Masaki Matsui at Hokkaido University’s Faculty of Science, have developed a new method to synthesize lithium cobalt oxide at temperatures as low as 300°C and durations as short as 30 minutes.
Their findings were published in the ACS journal Inorganic Chemistry.
Reaction pathway of the hydroflux process to form layered lithium cobalt oxide (LiCoO2) at 300 °C. (Illustration: Masaki Matsui)
Using cobalt hydroxide and lithium hydroxide as starting materials, with sodium or potassium hydroxide as an additive, the team conducted a series of experiments under varying conditions to synthesize layered LiCoO2 crystals. The process was called the hydroflux process. They were also able to determine the reaction pathway that led to the formation of the layered crystals.
Obvious crystal growth of LiCoO2 synthesized via hydroflux process compared with solid-state process at 300 °C by (a) XRD and (b) SEM observation. (Rannosuke Maeda, et al. Inorganic Chemistry.)
Molten mixed hydroxide-containing water molecules significantly accelerated the formation of LiCoO2, which showed a highly reversible capacity of 120 mAh g–1 without postannealing. The reaction mechanism study showed fast growth of LiCoO2 crystals, suggesting that the excess molten hydroxides containing water dissolve the cobalt species of HCoO2–. Consequently, the accelerated LiCoO2 formation suppresses the competing reaction of Co3O4 formation, leading to spinel LiCoO2 formation at low temperatures.
Excess water in the starting materials further accelerated the crystal growth of LiCoO2, forming large particles (>1 μm). Moreover, the layered LiCoO2 began to form at 150 °C.
This study is the first experimental demonstration that proves the thermodynamic stability of layered LiCoO2 at low temperatures (150–300 °C) under ambient pressure. This novel process offers significant energy savings in the production process of LiCoO2 and other ceramics materials.
—Maeda et al.
The team also measured the electrochemical properties of the layered LiCoO2, showing that they were only marginally inferior to that of commercially available LiCoO2 synthesized by the traditional high temperature method.
Resources
-
Rannosuke Maeda, Ryo Nakanishi, Minoru Mizuhata, and Masaki Matsui (2023) “Kinetically Enhanced Reaction Pathway to Form Highly Crystalline Layered LiCoO2 at Low Temperatures Below 300 °C” Inorganic Chemistry doi: 10.1021/acs.inorgchem.3c01704

Uranium Exploration Company Announces Additional Staking in the Athabasca Basin
Source: Streetwise Reports 12/22/2023
Skyharbour Resources Ltd. announced an update from its Canada-based Falcon Project along with additional…
Tesla Launches New Mega Factory Project In Shanghai, Designed To Manufacture 10,000 Megapacks Per Year
Tesla Launches New Mega Factory Project In Shanghai, Designed To Manufacture 10,000 Megapacks Per Year
Tesla has launched a new mega factory…
Giving thanks and taking stock after “a remarkable year”
An end-of-year thank you to our readers, industry colleagues and advertisers before Electric Autonomy breaks from publishing until Jan. 2
The post Giving…