Base Metals
Intellia, Verve run clinical trials abroad amid FDA’s ‘cautious’ approach to gene editing
Intellia Therapeutics announced earlier this month that it would run a mid-stage trial of a CRISPR-based treatment for a rare genetic disease called hereditary…

Intellia Therapeutics announced earlier this month that it would run a mid-stage trial of a CRISPR-based treatment for a rare genetic disease called hereditary angioedema (HAE) entirely outside of the US. The decision came after the FDA asked for more germline research if the trial was to include women of child-bearing age.
So far, at least two gene editing companies — Intellia and Verve Therapeutics — have decided to run early- or mid-stage clinical trials outside the US, as FDA takes a more cautious approach than foreign regulators to the nascent field of gene editing.
There’s palpable excitement around gene editing’s potential. These treatments are being developed at an unprecedented pace, reaching clinical trials less than a decade after CRISPR/Cas9 was first described as a gene editing technique by Jennifer Doudna and Emmanuelle Charpentier in 2012. For next-generation gene editing techniques like base editing — a method to make single-letter changes in a DNA sequence which was first described by David Liu in 2016 — progress has been even faster.
But that speed and innovation now have to come to terms with a more prudent regulator in the US, which requested more information from Intellia and also placed clinical holds on other gene editing treatments before they entered the clinic.
“We take very seriously when we put something on a clinical hold,” FDA CBER director Peter Marks said in a separate interview published Wednesday with Endpoints News. “We do so when we have scientific questions that remain about whether it will be safe to go ahead and treat individuals with the therapies in question.”
“There have been enough adverse issues with gene therapies. That has led us to be somewhat cautious,” Marks said.
More animal data
Intellia, which is co-founded by Nobel laureate Jennifer Doudna and headquartered in Cambridge, MA, received clearance to run the HAE study in the US in March, but that was followed by a request for additional germline data to include women of child-bearing age in the study. Instead of changing the study protocol to exclude this group of women or potentially delay the trial, Intellia announced Aug. 3 its plan to enroll all patients at sites that were already recruiting in Europe and New Zealand.
 John Leonard
John Leonard“Rather than delay, the idea was to complete the work, submit that to the FDA, and bring US patients into the study in Phase III,” Intellia CEO John Leonard said during the company’s most recent earnings call.
In July of last year, the FDA put a hold on the IND — the application to run a trial — for Beam Therapeutics’ base-edited CAR-T therapy, asking for more preclinical data. The FDA then lifted it six months later. (Beam has yet to dose a patient, though it says it plans to do so this quarter.)
And in November, the FDA put a hold on the IND for Verve’s hereditary heart disease treatment. The biotech continued to run its trials in New Zealand and the UK.
Verve, which licensed the base editing technology from Beam, said in its August financial report that it is still working on addressing the questions the FDA had about potential off-target and germline edits and now expects to complete enrollment of the trial outside the US.
‘Growing pains’
Gene editing treatments are still in the earlier stages of testing, and it’s unclear how regulatory caution and subsequent delays may affect their future development. The FDA’s caution is slowing things down, and that could delay when these treatments reach patients, according to Jefferies analyst Maury Raycroft in an interview, but that process will likely become more streamlined as the FDA learns what kind of information it needs from companies. He described the process as “growing pains.”
“It’s going to impact the space in a positive way, ultimately,” he said.
Raycroft also pointed out that the FDA initially took a conservative approach to RNAi treatments. He added that for many of the diseases, these gene editing companies developing treatments already have approved therapies.
The FDA has only cleared a handful of in vivo gene editing treatments for human trials, but a number of therapies have not panned out, including an Editas therapy for an eye disease, Sangamo’s zinc finger treatment for hemophilia B, and LogicBio’s candidate for methylmalonic acidemia. Intellia’s HAE treatment is the first cleared for testing in the US that has promising clinical data. Phase I data from 10 patients outside the US who received the one-time treatment showed a 95% reduction in the painful swelling attacks that are associated with the disease.
One early-stage gene-editing trial that is running in the US is expected to soon have initial data on a few patients. In 2021, the FDA cleared Excision Biotherapeutics to test its CRISPR treatment for HIV in men, and Excision dosed its first patient last year. Rather than editing a human gene, the therapy targets HIV viral DNA that has been integrated in human cells.
The real test of the FDA’s thinking will be whether the regulator clears the Phase III trial for Intellia’s other CRISPR treatment for ATTR amyloidosis, which is currently being tested in an early-stage study in France, New Zealand and Sweden.
Intellia has said it plans to design the trial to be similar to other late-stage ATTR amyloidosis trials run by companies like Alnylam, which developed an RNAi treatment for the disease where a toxic protein builds up in organs and nerves. But Myles Minter, an analyst at William Blair, said in an interview there is a “healthy level of skepticism” around whether the FDA will clear the trial. He noted the study will likely enroll around 600 patients, though no patients in the US have been treated with Intellia’s gene editing treatment so far.
Meanwhile, Verve plans to unveil initial ex-US data later this year on its heterozygous familial hypercholesterolemia base-editing treatment.
“It’s always hard to get compared to foreign regulators because we can always find places where they were probably ahead of us and right,” Marks said in the Q&A. “But we can also find places like thalidomide where they were ahead of us and wrong.”

White House Prepares For “Serious Scrutiny” Of Nippon-US Steel Deal
White House Prepares For "Serious Scrutiny" Of Nippon-US Steel Deal
National Economic Adviser Lael Brainard published a statement Thursday…
How to Apply for FAFSA
Students and families will see a redesigned FAFSA this year. Here’s how to fill it out.
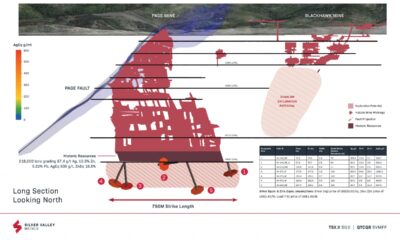
Dolly Varden consolidates Big Bulk copper-gold porphyry by acquiring southern-portion claims – Richard Mills
2023.12.22
Dolly Varden Silver’s (TSXV:DV, OTCQX:DOLLF) stock price shot up 16 cents for a gain of 20% Thursday, after announcing a consolidation of…