Energy & Critical Metals
Galera Therapeutics (NASDAQ: GRTX) in Focus: Exploring Catalysts Ahead of FDA’s Verdict
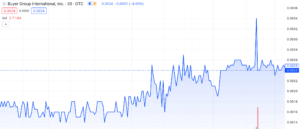
Galera Therapeutics (NASDAQ: GRTX) experienced a significant 34% intraday surge on October 18, 2023. This explosive trading has captured the attention…
Galera Therapeutics (NASDAQ: GRTX) experienced a significant 34% intraday surge on October 18, 2023. This explosive trading has captured the attention of investors, prompting curiosity about the company’s efforts to innovate radiotherapy in cancer treatment. Despite the lack of updates on social media, press releases, or SEC filings, this sudden rise has left many intrigued about the developments within the company. Stepping back to analyze the broader landscape can offer insights into potential future prospects and any groundbreaking advancements that might be on the horizon. Let’s delve deeper into the situation to grasp the overall trajectory and potential impactful developments in the near term.
Devastating Blow:
One of the first things you’ll notice about GRTX is trading substantially below Nasdaq’s minimum bid compliance and prior to today’s 34% gain, was sitting at a mere $0.22 per share. There’s of course good reason for this, and the details behind it are directly linked to a press release from August 9th, 2023.
According to the FDA, data from Galera’s phase 3 ROMAN trial and supporting GT-201 trial were not sufficiently persuasive to establish substantial evidence of avasopasem’s effectiveness and safety for reducing severe oral mucositis in patients with head and neck cancer.
The news caused an 82.6% drop in the company’s shares and coupled with the FDA’s concerns came a request for an additional trial. This of course was not ideal for Galera, and they immediately planned a substantial workforce reduction (around 70%) and is currently exploring partnerships for the drug’s development. Mentioned in the release is the companys intent to discuss the FDA’s decision and potential next steps for avasopasem’s approval.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
September Release:
In their recent update, Galera revealed plans for a crucial meeting with the FDA set for September 28, 2023. The focus of this meeting is to address the FDA’s Complete Response Letter (CRL) regarding Galera’s drug avasopasem manganese (avasopasem), designed to combat severe oral mucositis in head and neck cancer patients undergoing standard treatment.
Mel Sorensen, the CEO of Galera, emphasized their eagerness to comprehend the FDA’s perspective and data evaluation, optimistic about avasopasem’s potential in aiding those grappling with severe oral mucositis. The insights gained from this meeting will significantly influence the path forward, potentially paving the way for avasopasem’s approval and its positive impact on patients. This meeting stood as a pivotal and high-priority event in the drug’s developmental journey for the company.
Analyst Rating:
You might be curious about the value of this company prior to the FDA’s unfortunate news. Interestingly, one analyst, Tazeen Ahmad from the Bank of America, transformed his perspective, upgrading the company from an underperform to a buy rating, while also raising the price target from $2 to $7 back in February. This upgrade was primarily spurred by the FDA granting priority review status for Galera’s key asset, avasopasem.
It’s crucial to highlight the lack of FDA-approved therapies for severe oral mucositis (SOM), which represents a significant unmet need in the medical field. Preceding Ahmad’s upgrade, Galera had recently undertaken a $30 million fundraising effort, resulting in some dilution that triggered a selloff. Surprisingly, Ahmad saw this as an attractive buying opportunity.
Considering these factors, it becomes clearer how the potential upside could be substantial if the FDA provides a positive response to Galera in the near future. As we continue to delve deeper, we’ll discover just how quickly the FDA will be responding to Galera and what many online believe the opportunity at hand is.
Subscribe to Microcapdaily.com Right Now by entering your Email in the box below.
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Now What:
Key aspects of Galera moving forward are the upcoming key catalysts that have now sparked significant interest among online investors. What may not be widely known among the average investor is that the FDA feedback is fast approaching, with a deadline set for October 28, 2023. Online discussions have highlighted a notable resistance level at approximately $0.24. As the deadline approaches, more investors are keen to join the potential upward trend, especially considering the volatile performance seen in several other small-cap biotech companies with FDA feedback.
With the stock breaking a significant resistance point today on October 18th, 2023, there’s a growing belief online that it could potentially reach $0.50 or even higher by the end of the month, particularly if the FDA releases positive news. One user mentions a potential outcome could be the FDA permitting a resubmission of their NDA for full FDA approval. If that were the case, it could trigger substantial trading activity but it’s important to note this is highly speculative and comes with substantial risk.
There are even multiple comparisons being drawn between GRTX and TPST, the latter witnessing a remarkable ~4000% gain within a day after a short squeeze was triggered. To put it into perspective, a $1,000 investment would have skyrocketed to $40,000 if you bought and sold at the right time. If you’re intrigued by this event and would like to delve deeper to draw your own parallels, we recently covered it in detail, click here to review.
Conclusion:
If you didn’t have time to read through everything, here’s the TLDR; FDA approvals can trigger substantial gains for micro cap biotech stocks, especially those trading below a dollar with low floats (limited shares outstanding).
If the FDA gives the green light, there’s often notable upside. If you’re considering the potential benefits post-October 28th, be sure to do your own thorough research. Keep in mind, there are many things to consider in the world of clinical stage biopharma companies – it’s extremely diverse.
While many retail traders often YOLO an investment with potential positive news from the FDA, we always suggest digging deep to grasp the safety profile, therapeutic value, and market factors associated with any clinical stage drug.
We will update you on GRTX when more details emerge, subscribe to Microcapdaily to follow along!
Subscribe to Our 100% Free Penny Stock Newsletter. We Have Something Big Coming!
Disclosure: We have not been compensated for this article/video. MicroCap Daily is not an investment advisor; this article/video does not provide investment advice. Always do your research, make your own investment decisions, or consult with your nearest financial advisor. This article/video is not a solicitation or recommendation to buy, sell, or hold securities. This article/video is our opinion, is meant for informational and educational purposes only, and does not provide investment advice. Past performance is not indicative of future performance.
Picture by R-region from Pixabay
The post Galera Therapeutics (NASDAQ: GRTX) in Focus: Exploring Catalysts Ahead of FDA’s Verdict first appeared on Micro Cap Daily.
The post Galera Therapeutics (NASDAQ: GRTX) in Focus: Exploring Catalysts Ahead of FDA’s Verdict appeared first on Micro Cap Daily.

Uranium Exploration Company Announces Additional Staking in the Athabasca Basin
Source: Streetwise Reports 12/22/2023
Skyharbour Resources Ltd. announced an update from its Canada-based Falcon Project along with additional…
Tesla Launches New Mega Factory Project In Shanghai, Designed To Manufacture 10,000 Megapacks Per Year
Tesla Launches New Mega Factory Project In Shanghai, Designed To Manufacture 10,000 Megapacks Per Year
Tesla has launched a new mega factory…
Giving thanks and taking stock after “a remarkable year”
An end-of-year thank you to our readers, industry colleagues and advertisers before Electric Autonomy breaks from publishing until Jan. 2
The post Giving…