Base Metals
Stanford researchers make ammonia from air and water microdroplets
Stanford researchers, with a colleague from King Fahd University of Petroleum and Minerals, have developed a simple and environmentally sound way to make…
Stanford researchers, with a colleague from King Fahd University of Petroleum and Minerals, have developed a simple and environmentally sound way to make ammonia with tiny droplets of water and nitrogen from the air. An open-access paper on their work is published in Proceedings of the National Academy of Sciences (PNAS).
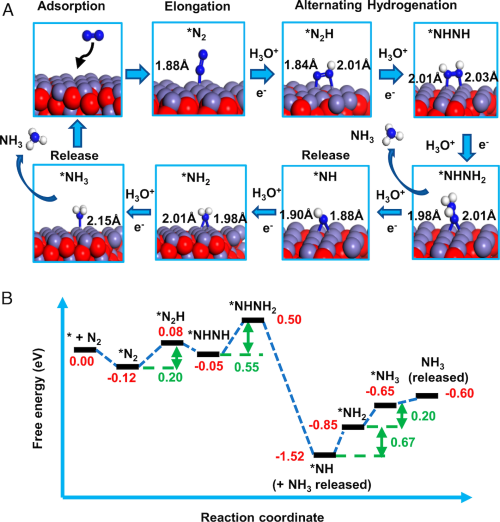
Water (H2O) microdroplets are sprayed onto a magnetic iron oxide (Fe3O4) and Nafion-coated graphite mesh using compressed N2 or air as the nebulizing gas. The resulting splash of microdroplets enters a mass spectrometer and is found to contain ammonia (NH3). This gas–liquid–solid heterogeneous catalytic system synthesizes ammonia in 0.2 ms.
The conversion rate reaches 32.9 ± 1.38 nmol s−1 cm−2 at room temperature without application of an external electric potential and without irradiation.
Water microdroplets are the hydrogen source for N2 in contact with Fe3O4. Hydrazine (H2NNH2) is also observed as a by-product and is suspected to be an intermediate in the formation of ammonia. This one-step nitrogen-fixation strategy to produce ammonia is eco-friendly and low cost, which converts widely available starting materials into a value-added product.
—Song et al.
Catalytic mechanism for ammonia formation from N2 and water droplets striking Fe3O4. (A) Diagrams of the reaction steps and (B) corresponding free energy changes found from DFT calculations. Song et al., PNAS
For more than a century, the world has relied on the Haber-Bosch process to yield ammonia in bulk; however, the industrial procedure is energy intensive. To break nitrogen’s strong bonds, the Haber-Bosch process requires roughly 80-300 atmospheres of pressure and temperatures around 572-1000 ˚F (300-500 ˚C). The steam-treating of natural gas involved in the process also releases ample amounts of carbon dioxide.
All told, to satisfy the current annual worldwide demand for 150 million metric tons of ammonia, the Haber-Bosch process consumes more than 2% of global energy and accounts for about 1% of the carbon dioxide emitted into the atmosphere.
In contrast, the innovative method debuted by the Stanford researchers requires less specialized circumstances.
We were shocked to see that we could generate ammonia in benign, everyday temperature-and-pressure environments with just air and water and using something as basic as a sprayer. If this process can be scaled up, it would represent an eco-friendly new way of making ammonia, which is one of the most important chemical processes that takes place in the world.
—senior author Richard Zare
The new method also uses little energy and at low cost, thus pointing a way forward to potentially producing the valuable chemical in a sustainable manner. Xiaowei Song, a postdoctoral scholar in chemistry at Stanford, is the lead author of the study.
The new chemistry discovered follows pioneering work by Zare’s lab in recent years examining the long-overlooked and surprisingly high reactivity of water microdroplets. In a 2019 study, Zare and colleagues novelly demonstrated that caustic hydrogen peroxide spontaneously forms in microdroplets in contact with surfaces. Experiments since have borne out a mechanism of electric charge jumping between the liquid and solid materials and generating molecular fragments, known as reactive oxygen species.
While promising, the ammonia production method is only at the demonstration stage. The researchers plan to explore how to concentrate the produced ammonia as well as gauge how the process could potentially be scaled up to commercially viable levels.
While Haber-Bosch is only efficient when pursued at huge facilities, the new ammonia-making method could be portable and done on-site or even on-demand at farms. That, in turn, would slash the greenhouse gas emissions related to the transportation of ammonia from far-off factories.
Resources
-
Xiaowei Song, Chanbasha Basheer and Richard N. Zare (2023) “Making ammonia from nitrogen and water microdroplets” PNAS doi: 10.1073/pnas.2301206120

White House Prepares For “Serious Scrutiny” Of Nippon-US Steel Deal
White House Prepares For "Serious Scrutiny" Of Nippon-US Steel Deal
National Economic Adviser Lael Brainard published a statement Thursday…
How to Apply for FAFSA
Students and families will see a redesigned FAFSA this year. Here’s how to fill it out.
Dolly Varden consolidates Big Bulk copper-gold porphyry by acquiring southern-portion claims – Richard Mills
2023.12.22
Dolly Varden Silver’s (TSXV:DV, OTCQX:DOLLF) stock price shot up 16 cents for a gain of 20% Thursday, after announcing a consolidation of…